
Kaushik Biswas
Professor
Kaushik Biswas
Professor, Biological Sciences
PhD: Indian Institute of Chemical Biology, 2001
Previous appointments:
Previous Positions
2015-2020 : Associate Professor, Div. of Molecular Medicine, Bose Institute, Kolkata
2010-2015 : Assistant Professor, Div. of Molecular Medicine, Bose Institute, Kolkata
2003-2010 : Post Doctoral Research Fellow, Cleveland Clinic, Cleveland, Ohio, USA
Research interests:
LABORATORY OF TUMOR GLYCOBIOLOGY
Understanding the mechanism of tumorigenesis specifically focusing on the role of glycolipids in tumor growth, progression and metastasis.
Specific Research Focus :
How tumor derived Gangliosides modulate tumor growth, progression and metastasis ?
Targeted Genome Editing tools (TALEN and CRISPR) to study mechanisms in carcinogenesis, focusing primary on unknown functions of genes in cancer
Defining the Epigenetic Landscape underlying the Regulation of ganglioside synthase genes in cancer
Potential Role of microRNAs in tumorigenesis
How Tumor Microenvironment contributes to metastasis ?
For More Information, please visit my Homepage ( www.tumorglycobiolab.in )
Contact:
| Address: |
Biological Sciences Unified Academic Campus Bose Institute EN-80, Sector V Bidhan Nagar Kolkata - 700 091, India |
| E-Mail: | kaushik[at]jcbose.ac.in |
| Phone: | +91-33-25693260 |
Research:
We ask two basic questions - Why select gangliosides are over-expressed in certain cancers ? and What's the consequence of this over-expression in relation to tumorigenesis ? To adress these, we have taken an all-round approach. Gene silencing as well as molecular cloning and over-expression strategies are utilized to establish the potential role of tumor derived glycosphingolipids in migration as well as invasion of tumor cells. Gene expression profiling suggests a large number of differentially regulated gene sets (DRGs) in response to GM2-synthase knockdown and mechanistic studies reveal a critical role of the integrin signaling pathway to be involved in GM2 mediated tumor cell migration. With an aim to translate these in vitro findings in vivo, targeted genome editing technology by TALEN, as well as CRISPR-Cas9 is used to define the functional role of GM2 in tumorigenesis. Studies indicate that GM2 is involved in epithelial-mesenchymal transition (EMT) by promoting anoikis resistance in tumors. Studies are under way to elucidate the mechanism of GM2 mediated EMT. Finally, we have initiated a study to find out the basis of over-expression of several ganglioside synthase genes in cancer, currently focusing on the regulation of GM2-synthase gene. Very recently, data from our lab showed that the GM2-synthase gene is epigenetically regulated in renal cell carcinoma (RCC or kidney cancer) which may well extend to other cancers as well. In this recent finding, we uncovered a novel mechanism regulating the expression of this oncogene, GM2-synthase at the transcriptional level. On a different note, recent data indicate a potential role of micro RNA in GM2-mediated tumorigenesis.
Publications:
Khamrui, E., Banerjee, S., Mukherjee, D.D. and Biswas, K*. Emerging role of MAPK signaling in glycosphingolipid-associated tumorigenesis. Glycoconjugate Journal, Oct 5, 2024, part of a collection “Tribute to Professor Sen-Itiroh Hakomori“, https://rdcu.be/dV8RK, https://doi.org/10.1007/s10719-024-10168-5.
Ray, A., Sarkar, A., Banerjee, S. and Biswas, K*. Non-Canonical Targets of MicroRNAs: Role in Transcriptional Regulation, Disease Pathogenesis and Potential for Therapeutic Targets. MicroRNA, 13(2), 83-95, Jan 22, 2024, 10.2174/0122115366278651240105071533.
Sarkar, A., Banerjee, S. and Biswas, K.* Multi-dimensional role of gangliosides in modulating cancer hallmarks and their prospects in targeted cancer therapy. Frontiers Pharmacol., 14, 2023, https://doi.org/10.3389/fphar.2023.1282572.
Debnath, S., Sarkar, A., Mukherjee, D.D., Ray, S., Mahata, B., Mahata, T., Parida, P.K., Das, T., Mukhopadhyay, R., Ghosh, Z. and Biswas, K.* Eriodictyol mediated selective targeting of the TNFR1/FADD/TRADD axis in cancer cells induce apoptosis and inhibit tumor progression and metastasis. Translational Oncology, 21, July 2022.
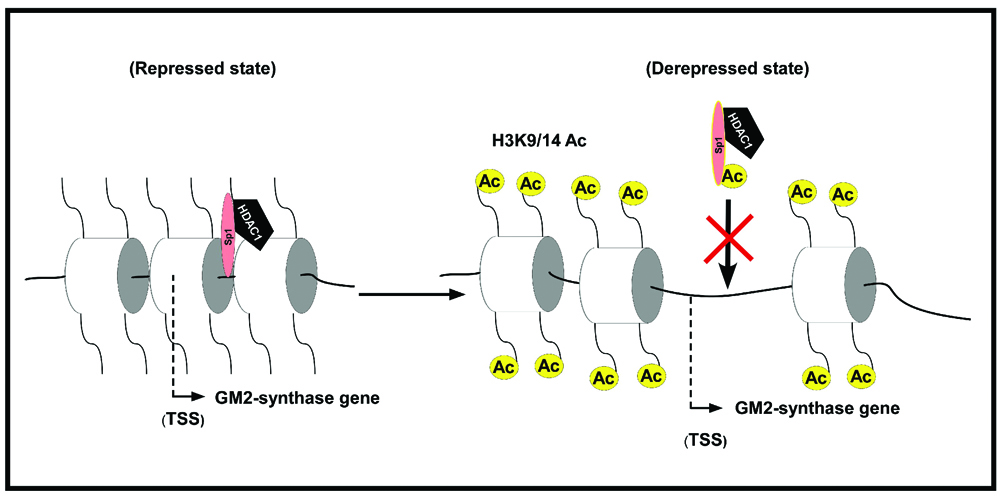
Banerjee, A., Mahata, B., Dhir, A., Mandal, T.K. and Biswas, K.* Elevated histone H3 acetylation and loss of the Sp1-HDAC1 complex de-repress the GM2-synthase gene in renal cell carcinoma. J. Biol. Chem., 294(3), 1005-1018 (2019).
Dhabal, S., Das, P., Biswas, P., Kumari, P., Yakubenko, V.P., Kundu, S., Cathcart, M.K., Kundu, M., Biswas, K. and Bhattacharjee, A.* "Regulation of monoamine oxidase A (MAO-A) expression, activity, and function in IL-13-stimulated monocytes and A549 lung carcinoma cells." J. Biol. Chem., 293(36), 14040-14064 (2018).
Parida, P.K., Mahata, B., Santra, A., Chakraborty, S., Ghosh, Z., Raha, S., Misra, A.K., Biswas, K.* and Jana, K.* (*Joint corresponding author) Inhibition of cancer progression by a novel trans-stilbene derivative through disruption of microtubule dynamics, driving G2/M arrest, and p53-dependent apoptosis. Cell Death Dis., 9(5), 448 (2018).
Kundu, M, Mahata, B, Banerjee, A, Chakrabarty, S, Debnath, S, Sinha Ray, S, Ghosh, Z and Biswas, K.* Ganglioside GM2 mediates migration of tumor cells by interacting with integrin and modulating the downstream signaling pathway. Biochim Biophys Acta – Mol Cell Res., 1863 (7 Pt A), 1472-1489 (2016).
Mahata, B and Biswas, K*. Generation of stable knockout mammalian cells by TALEN mediated locus specific gene editing. Methods Mol Biol., 1498, 107-120 (2017).
Mahata, B, Banerjee, A, Kundu, M, Bandyopadhyay, U and Biswas, K.* TALEN mediated targeted editing of GM2/GD2-synthase gene modulates anchorage independent growth by reducing anoikis resistance in mouse tumor cells. Scientific Reports, 5 : 9048, 1-12 (2015).
Mahata, B, Biswas, S, Rayman, P, Chahlavi, A, Ko, J, Bhattacharjee, A, Li, YT, Li, Y, Das, T, Sa, G, Raychaudhuri, B, Vogelbaum, MA, Tannenbaum, C, Finke, JH and Biswas, K.* GBM Derived Gangliosides Induce T Cell Apoptosis through Activation of the Caspase Cascade Involving Both the Extrinsic and the Intrinsic Pathway. PLoS One, 10, 1-19 (2015).
Parida, PK, Sau, A, Ghosh, T, Jana, K, Biswas, K, Raha, S and Misra, AK. Synthesis and evaluation of triazole linked glycosylated 18β-glycyrrhetinic acid derivatives as anticancer agents. Bioorganic & Medicinal Chemistry Letters, 24, 3865-3868 (2014).
Biswas, K, Richmond, A, Rayman, P, Biswas, S, Thornton, M, Sa, G, Das, T, Zhang, R, Chahlavi, A, Tannenbaum, CS, Novick, A, Bukowski, R. and Finke, JH. GM2 Expression in Renal Cell Carcinoma : Potential Role in Tumor Induced T cell Dysfunction. Cancer Res, 66, 6816-6825 (2006).
Biswas, K, Bandyopadhyay, U, Chattopadhyay, I, Varadaraj, A, Ali, E. and Banerjee, RK. A novel antioxidant and antiapoptotic role of omeprazole to block gastric ulcer through scavenging of hydroxyl radical. J Biol Chem, 278, 10993-11001 (2003).
Bandyopadhyay, U, Biswas, K, Sengupta, A, Moitra, P, Dutta, P, Sarkar, D, Debnath, P, Ganguly, C.K. and Banerjee, RK. Clinical studies on the effect of Neem (Azadirachta indica) bark extract on gastric secretion and gastroduodenal ulcer. Life Sci, 75, 2867-2878 (2004).
Biswas, S, Richmond, A, Biswas, K, Ko, J, Ghosh, S, Simmons, M, Rayman, P, Rini, B, Gill, I, Tannenbaum, C.S. and Finke, JH. Elevated levels of select gangliosides in T cells from Renal Cell Carcinoma patients is associated with T-cell dysfunction. J. Immunol, 183(8), 5050-5058 (2009).
Chattopadhyay, I, Bandyopadhyay, U, Biswas, K, Maity, P and Banerjee, RK. Indomethacin inactivates gastric peroxidase to induce reactive-oxygen mediated gastric mucosal injury and curcumin protects it by preventing peroxidase inactivation and scavenging reactive oxygen. Free Radical Biology and Medicine, 40, 1397-1408 (2006).
Chahlavi, A, Rayman, P, Richmond, AL, Biswas, K, Zhang, R, Vogelbaum, M, Tannenbaum, C, Barnett, G and Finke, JH. Glioblastomas induce T-lymphocyte death by two distinct pathways involving gangliosides and CD70. Cancer Res, 65(12), 5428-38 (2005).
Raval, G, Biswas, S, Rayman, P, Biswas, K, Sa, G, Ghosh, S, Thornton, M, Hilston, C, Bukowski, R, Finke, JH and Tannenbaum, CS. “TNF-a Induction of GM2 Expression on Renal Cell Carcinomas Promotes T cell Dysfunction. J.Immunol, 178, 6642-6652 (2007).
Rayman, P, Wesa, AK, Richmond, AL, Das, T, Biswas, K, Raval, G, Storkus, WJ, Tannenbaum, CS, Novick, A, Bukowski, R and Finke, JH. Effect of Renal Cell Carcinomas on the Development of Type1 T-Cell Responses. Clinical Cancer Research, 10 (18Pt2), 6360S-6366S (2004).
Chattopadhyay, I, Nandi, B, Chatterjee, R, Biswas, K, Bandyopadhyay, U and Banerjee, RK. Mechanism of antiulcer effect of Neem (Azadirachta indica) leaf extract : effect on H+-K+-ATPase, oxidative damage and apoptosis. Inflammopharmacology, 12, 153-176 (2004).
Biswas, K, Chatterjee, I, Banerjee, RK and Bandyopadhyay, U. Biological activity and medicinal uses of Neem (Azadirachta indica). Current Sci, 82, 1336-1345 (2002).
Bandyopadhyay, U, Biswas, K, Chatterjee,R, Bandyopadhyay, D, Chattopadhyay, I, Ganguly, CK, Chakroborty, T, Bhattacharya, K and Banerjee, RK. Gastroprotective effect of Neem (Azadirachta indica) bark extract through inhibition of H+-K+-ATPase and scavenging of hydroxyl radical. Life Sci, 71, 2845-2865 (2002).
View MoreRecognition:
- Active Member, American Association for Cancer Research (AACR), from 2016
- Elected Fellow of the West Bengal Academy of Science & Technology (WAST), January 2022
Teaching:
For Integrated MSc PhD Course
Coordinator, Cell Biology
Coordinator, Molecular and Cellular Biology
PhD Course Work
Coordinator, Cell Signaling and Molecular Medicine
Students:
| Image | Name | Designation | Department | Campus | Contact number | |
|---|---|---|---|---|---|---|
 |
Abhisek Sarkar | Senior Research Fellow(I) | Division of Molecular Medicine | Centenary | abhiseksarkar1@gmail.com | |
 |
Aishwarya Ray | Junior Research Fellow | Division of Molecular Medicine | Centenary | aishwarya.dgp@gmail.com | |
 |
Sanchari Chatterjee | Junior Research Fellow | Division of Molecular Medicine | Centenary | sanchari.3009@gmail.com | |
 |
Sounak Banerjee | Junior Research Fellow | Division of Molecular Medicine | Centenary | sounakbanerjee@jcbose.ac.in | |
 |
Subha Ray | Junior Research Fellow | Division of Molecular Medicine | Centenary | subharay@jcbose.ac.in |
Former:
PRESENT GROUP MEMBERS
Abhisek Sarkar - Senior Research Fellow (SRF)
Elora Khamrui - Senior Research Fellow (SRF)
Sounak Banerjee - Senior Research Fellow (SRF)
Subha Ray - Senior Research Fellow (SRF)
Aishwarya Ray - Junior Research Fellow (JRF)
Sanchari Chatterjee - Junior Research Fellow (JRF)

LABORATORY ALUMNI
Dr. Dipanwita Mukherjee - Research Associate (DST-SERB NPDF)
Dr. Manjari Kundu - Post Doctoral Fellow (NIH, MARYLAND, USA)
Dr. Barun Mahata - Post Doctoral Fellow (RICE UNIVERSITY, TEXAS, USA)
Dr. Avisek Banerjee - Post Doctoral Fellow (YALE UNIVERSITY SCHOOL OF MEDICINE, USA)
Dr. Shibjyoti Debnath - Postdoctoral Research Fellow (DUKE UNIVERSITY, NC, USA)
Group News:
POSITIONS OPEN FOR PhD STUDENTS
Interested
Students may apply for the Projects below :
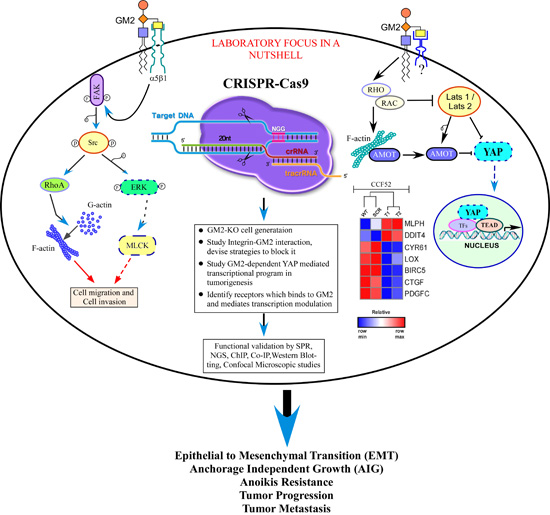
PROJECT
1 : Identifying the Upstream Components/Receptors involved in Ganglioside
GM2-mediated Regulation of the Hippo-YAP/TAZ axis in EMT and Metastasis.
Brief
Description - Recent reports from our
laboratory have established a potential role of the tumor derived
glycosphingolipid, GM2 in mediating epithelial-mesenchymal transition (EMT)
leading to tumor growth and progression. Preliminary data indicates
GM2-mediated inactivation of the critical tumor suppressor pathway, HIPPO and
subsequent de-phosphorylation induced nuclear translocation of YAP/TAZ leading
to upregulation of the YAP/TAZ-dependent genes, namely, ctgf, cyr61, lox, thbs1 etc. However, the precise signaling and
the molecular mechanism leading to GM2-mediated de-activation of the HIPPO
signaling is still unknown. Additionally, whether GM2-mediated HIPPO-YAP/TAZ
signaling translates functionally to induce EMT and metastasis needs to be
investigated. We hypothesize that GM2-dependent de-activation of HIPPO kinases,
namely Mst’s and LATs might involve receptor-dependent or independent
mechanisms at the cell surface, identification of which will provide an option
to regulate GM2-mediated tumorigenesis.
Requirement
- Post graduate in any discipline of Life Sciences or Biological Sciences
PROJECT 2 : How tumor-stromal interactions mediated by ganglioside GM2 contributes to metastasis ?
Brief Description - Tumor-stromal crosstalk shapes the metastatic landscape through mechanisms that are not completely understood. Fibroblasts form an important constituent of the tumor stroma, association of which with the tumor cells have been implicated in metastasis. Interesting data from our laboratory has shown that GM2 promotes cancer associated fibroblasts (CAF) formation. We intend to undertake a study to explore whether GM2 imparts CAF signatures in fibroblasts and thereby contributes in tumor metastasis.
Requirement - Post graduate in any discipline of Life Sciences, Biological Sciences with a knowledge and keen interest in Bioinformatics, or postgraduate in Bioinformatics/related field with knowledge and keen interest to learn modernbiological wet laboratory techniques.
PROJECT 3 : Biochemical and Structural Characterization of GM2-synthase.
Brief Description - Earlier research has attempted to elucidate the molecular structure of B4GALNT1 enzymes by using cloning and mutation approaches. However, deeper understanding of the molecular structure and detailed biochemistry of GM2 synthase is essential to elucidate its precise mechanism of action in ganglioside metabolism. Despite progress in identifying its functional domains and general catalytic role, the structural details remain poorly characterized. Future studies utilizing diverse cloning and mutational approaches, advanced techniques such as cryo-electron microscopy (cryo-EM), X-ray crystallography, and NMR spectroscopy could provide high-resolution insights into its three-dimensional architecture toward an in-depth understanding GM2-associated disease mechanisms, especially cancer and other neurodegenerative conditions. Comprehensive structural studies could also pave the way for the potential targeted therapies through modulate GM2 synthase activity.
Requirement - Post graduate in any discipline of Life Sciences, Biological Sciences with a knowledge and keen interest in Biochemistry, Molecular Biology and Protein Purification.- Feb 21, 2022 - Dipanwita Mukherjee was awarded the DST-SERB NPDF award
- July 01, 2021 - Aishwarya Ray, joined the Biswas Lab as a JRF
- June 23, 2021 - Sanchari Chatterjee, joined the Biswas Lab as a JRF
- August 16, 2019 - Avisek Banerjee was awarded his PhD degree from the University of Calcutta
- Sometime in 2019 - Avisek Banerjee left for a postdoctoral position in Yale University School of Medicine, USA.
- Dec 07, 2018 - Avisek Banerjee submitted his PhD thesis titled "Defining The Epigenetic Regulation of Ganglioside Synthase Genes in Cancer".
- Nov 30, 2018 - Manjari Kundu left for a postdoc position in TMC, Kolkata
- Oct 30, 2018 - Barun Mahata left for postdoc in RICE UNIVERSITY, Texas, USA
- Aug 01, 2018 - Sounak Banerjee joined as Junior Research Fellow (UGC)
- Aug 01, 2018 - Subha Ray joined as Junior Research Fellow (UGC)
- Sep 01, 2017 - Elora Khamrui joined as Junior Research Fellow (CSIR)
- June 12, 2017 - Barun Mahata joined as Senior Research Fellow (SRF extended, project)
- May 19, 2017 - Abhisek Sarkar joined as Junior Research Fellow (UGC)
- Apr 28, 2017 - Barun Mahata ( SRF) submitted his PhD thesis titled "Study the role and elucidate the mechanism of tumor derived gangliosides in tumor growth, progression and metastasis in syngenic mouse tumor model"
- Apr 01, 2017 - Manjari Kundu (ext SRF) defended her PhD thesis Viva Voce Examination
- Feb 18, 2017 - Barun Mahata (SRF) awarded the best poster award (1st Prize) at the 20th ADNAT convention held at KIIT, Bhubaneswar during Feb 16-18, 2017
- Jan 10, 2017 - Avisek Banerjee (SRF) awarded best poster award at the 3rd International Conference on PCSMM held at Bose Institute, Kolkata during Jan 8-10, 2017
- Aug 22, 2016 - Manjari Kundu (ext SRF) submitted her PhD thesis titled "Deciphering the role and elucidating the mechanism of tumor derived gangliosides in tumor cell growth, migration, invasiveness and metastasis"
- Nov 30, 2015 - Barun Mahata (SRF) awarded Professor B.B. Biswas Outstanding Student Award 2015
- March 25, 2014 - Shibjyoti Debnath joined as Junior Research Fellow (UGC Adhoc)