Basudeb Maji
Assistant Professor
Basudeb Maji
Assistant Professor, Biological Sciences
PhD: Indian Institute of Science, 2013
Previous appointments:
Employment
Assistant Professor
2022- Department of Biological Sciences, Bose Institute
2020-22 Trivedi School of Biosciences, Ashoka University
Department of Chemistry, Ashoka University
Postdoctoral Fellow
2015-20 Harvard Medical School
Broad Institute of MIT and Harvard
2016-17 Massachusetts Institute of Technology, Department of Chemistry
Research Associate
2013-15 Indian Institute of Science
Research interests:
Please visit us on https://www.bmajinative.com/
We aspire to translate the basic research into therapeutic applications.
Currently, we are focusing on the following research areas
1. Organic Synthetic Small Molecules and Anticancer Drug Development
We work on developing synthetic small molecules and chemical biology methods for developing targeted chemotherapy. We employ small-molecule probes to decipher the role of various DNA-binding proteins or transcription factors in genome stability, repair, plasticity, oncogenesis, and tumor progression
2. CRISPR-Cas, Genome engineering, Functional Genomics
We focus on developing functionally enhanced CRISPR-systems for their broader and practical application in gene-editing. We employ protein-engineering and chemogenetics for precise cellular gene-editing and investigate their effect on gene-functions for prospective gene-therapy applications
3. Synthetic Biology and Anti-infective Drug Development
We are working on designing synthetic biology methods for developing precision anti-infective agents. Our primary targets are multidrug-resistant pathogenic bacteria like MRSA and Mycobacterium tuberculosis
Contact:
| Address: |
Biological Sciences Unified Academic Campus Bose Institute EN-80, Sector V Bidhan Nagar Kolkata - 700 091, India |
| E-Mail: | bmaji[at]jcbose.ac.in |
Research:
Dr. Basudeb Maji’s research group (Chemogenetic Research Lab) at Bose Institute is working on the frontier of disease biology chemical biology, and medicinal chemistry problems. His lab mainly focuses on protein-engineering, genome engineering, and chemogenetics for precision gene therapy applications and chemopreventive development. His lab works on both organic synthesis and synthetic biology for developing synthetic small molecular probes for genetic and pathogenic diseases like cancer, muscular disorders, and bacterial infection.
Publications:
Publications
For an updated list, please follow the Google Scholar link
1.Design and Synthesis of
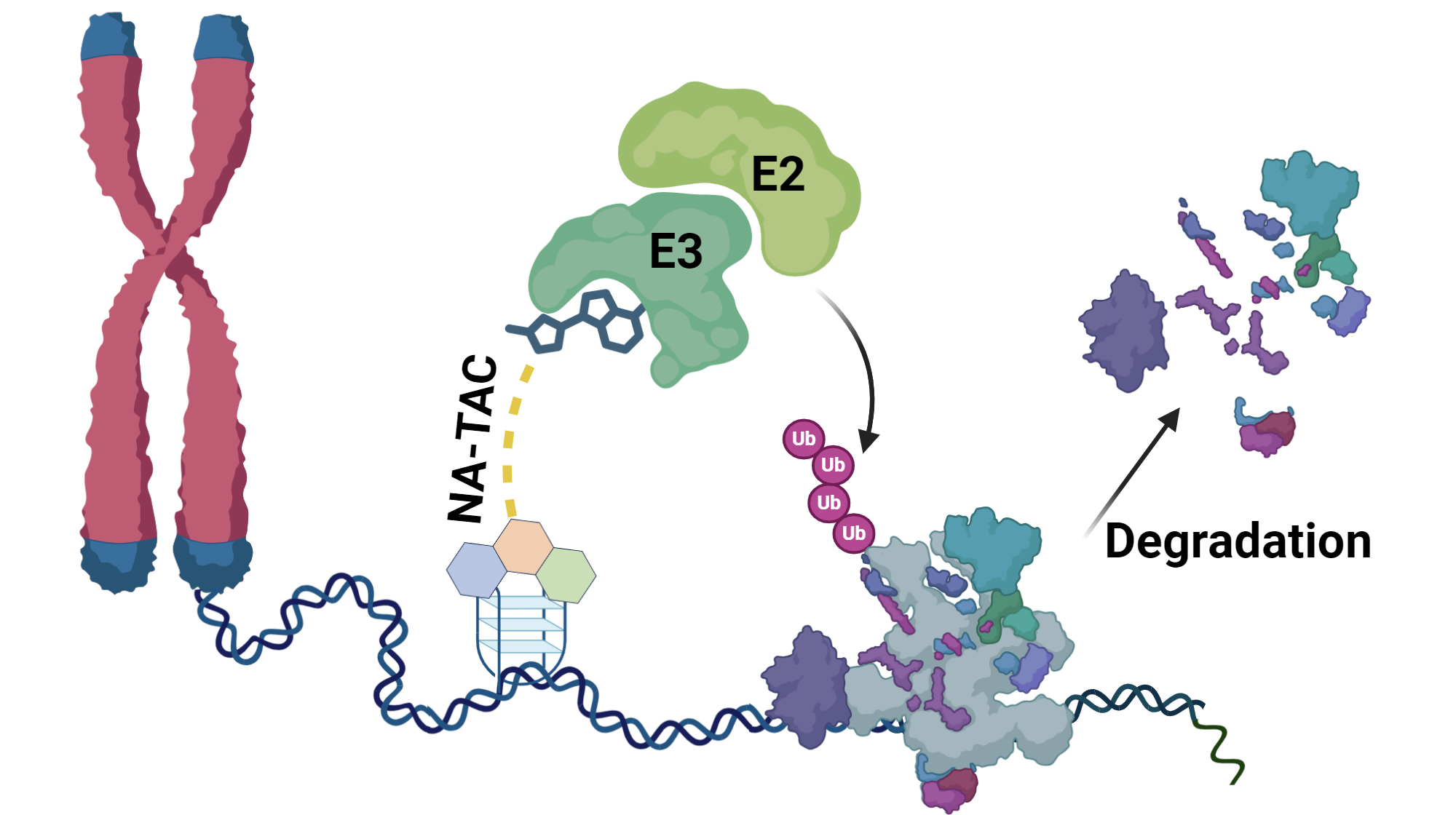
Nucleic Acid Nano-environment Interactome-Targeting Small Molecule PROTACs and
Their Anticancer Activity. Sadiya Tangaa, Arkadeep Karmakarb, Arpita
Hotab, Paramita Banerjeec and Basudeb Maji. Nanoscale, 2024, In press.
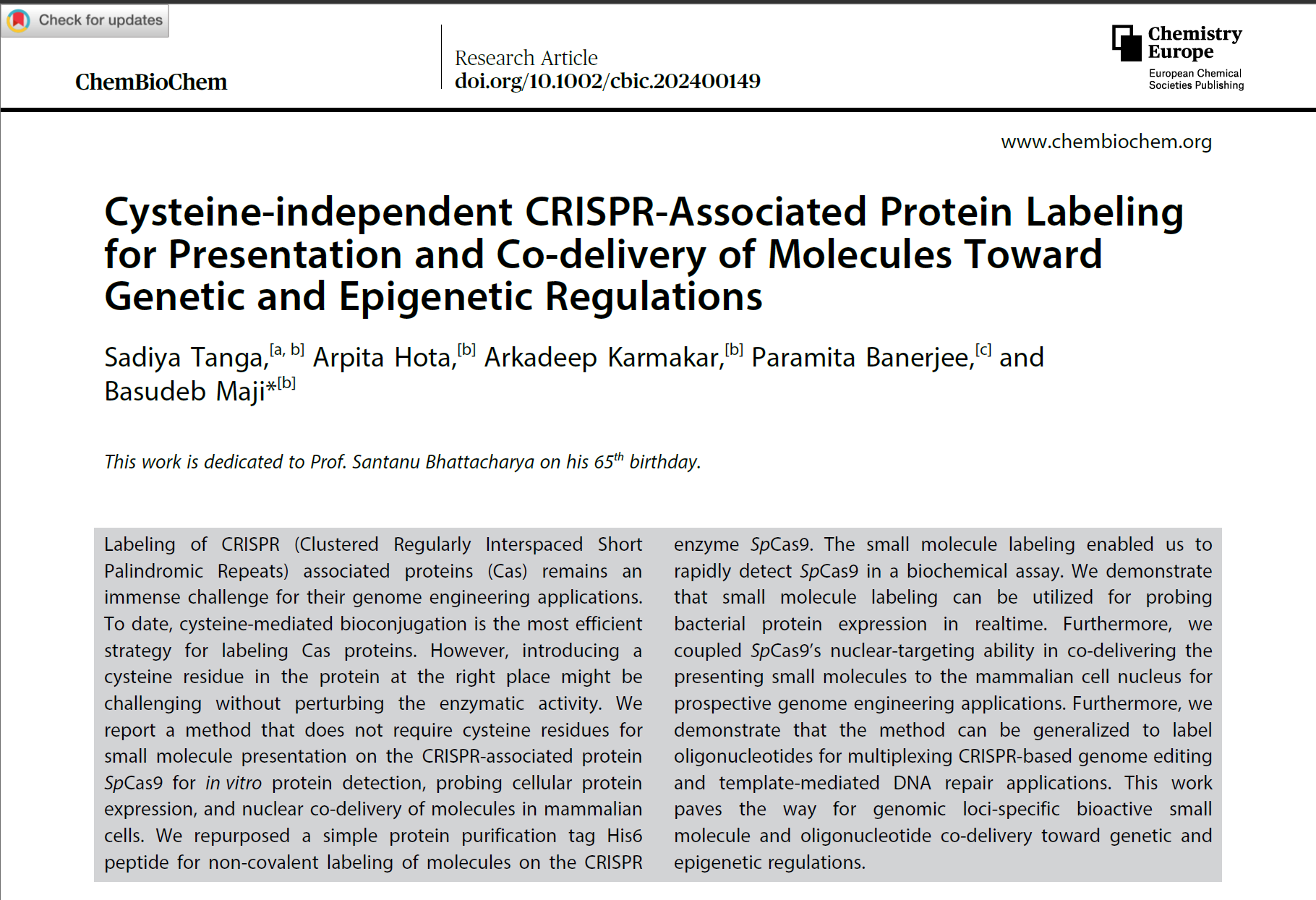
2. Cysteine-independent CRISPR-associated protein labeling for presentation and co-delivery of molecules toward genetic and epigenetic regulations. ChemBioChem, 2024, Sadiya Tanga, Arpita Hota, Arkadeep Karmakar, Paramita Banerjee, and Basudeb Majib,* In press, http://dx.doi.org/10.1002/cbic.202400149.
3. Inhibitors of RNA guided nucleases and uses thereof A Choudhary, P Wu, B Maji, E Franco, HKK SubramanianUS Patent 11,787,795

4. Nucleic acid editing (Nucleic acid biology and its application in human diseases): Springer Nature. Ayush Mistry, Sadiya Tanga, Basudeb Maji. 2023, https://link.springer.com/chapter/10.1007/978-981-19-8520-1_11.
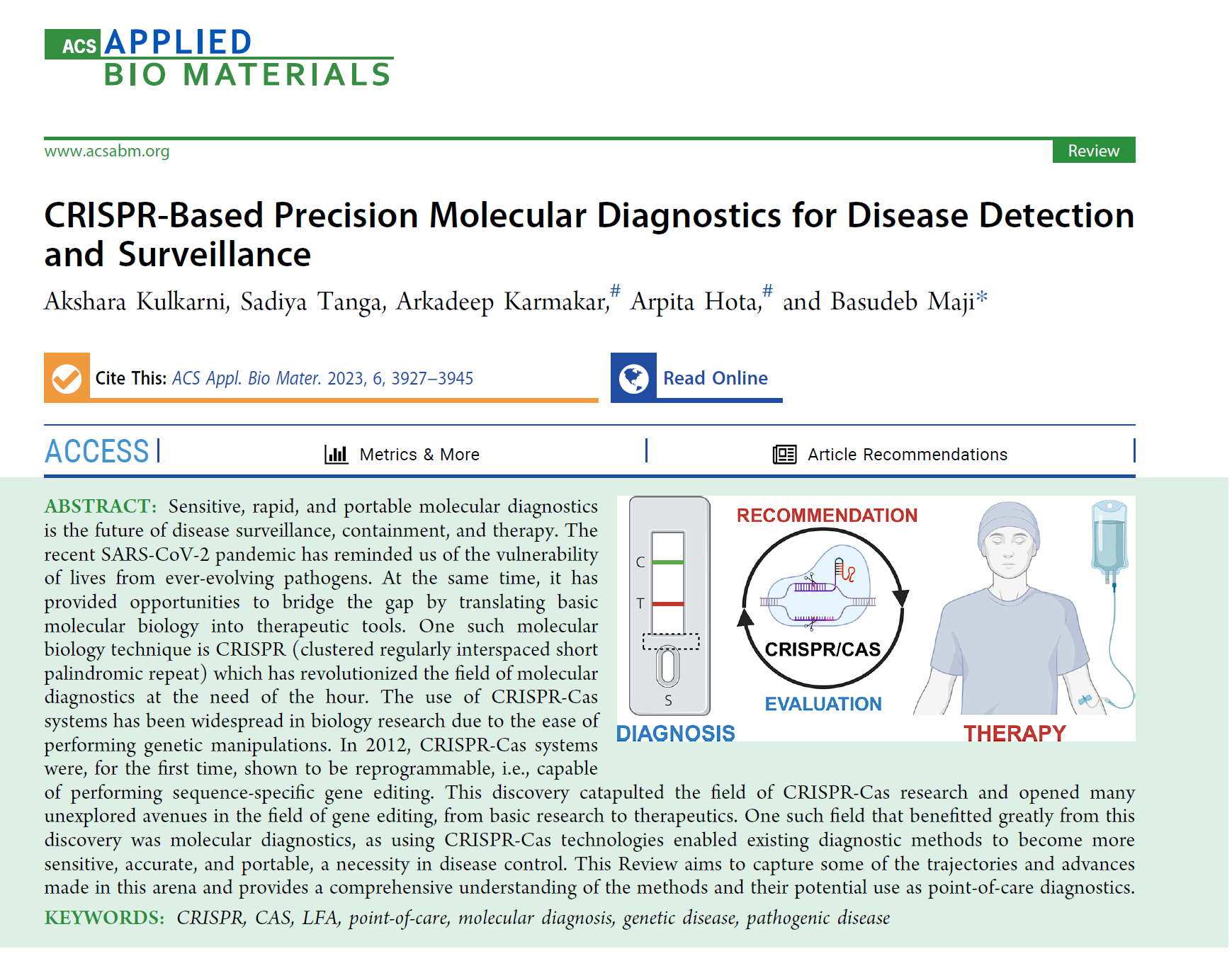
5. CRISPR-based Precision Molecular Diagnostics for Disease Detection and Surveillance. Akshara Kulkarni, Sadiya Tanga, Arkadeep Karmakar, Arpita Hota, and Basudeb Maji, 2023, ACS Applied Bio Materials, 2023, 6, 10, 3927–3945. https://doi.org/10.1021/acsabm.3c00439
6. CRISPR protein inhibitors A Choudhary, K Cox, B Maji, P Wu, H Subramanian, E Franco US Patent 11,760,984
9. Kei Yamada+, Arghya Deb+, Veronika M. Shoba, Donghyun Lim, Basudeb Maji, Ashley E. Modell, and Amit Choudhary*. Rational Design of Silicon-Based Zinc Ionophores with Antibacterial Activity. Angew. Chem. Int., 2022, 134 (23), e202201698. (IF 15.3).
10. A general approach to identify cell-permeable, miniature, and synthetic anti-CRISPRs. Donghyun Lim, Qingxuan Zhou, Kurt Cox, Benjamin Law, Miseon Lee, Praveen Kokkonda, Santosh Chaudhary, Rajaiah Pergu, Vedagopuram Sreekanth, Soumyashree Gangopadhyay, Basudeb Maji, Sophia Lai, Yuka Amako, David Thompson, Hari Subramanian, Michael Mesleh, Vlado Dančík, Paul Clemons, Bridget Wagner, Christina Woo, and George Church, Amit Choudhary. Nature Cell Biology, 2022, 24, 1
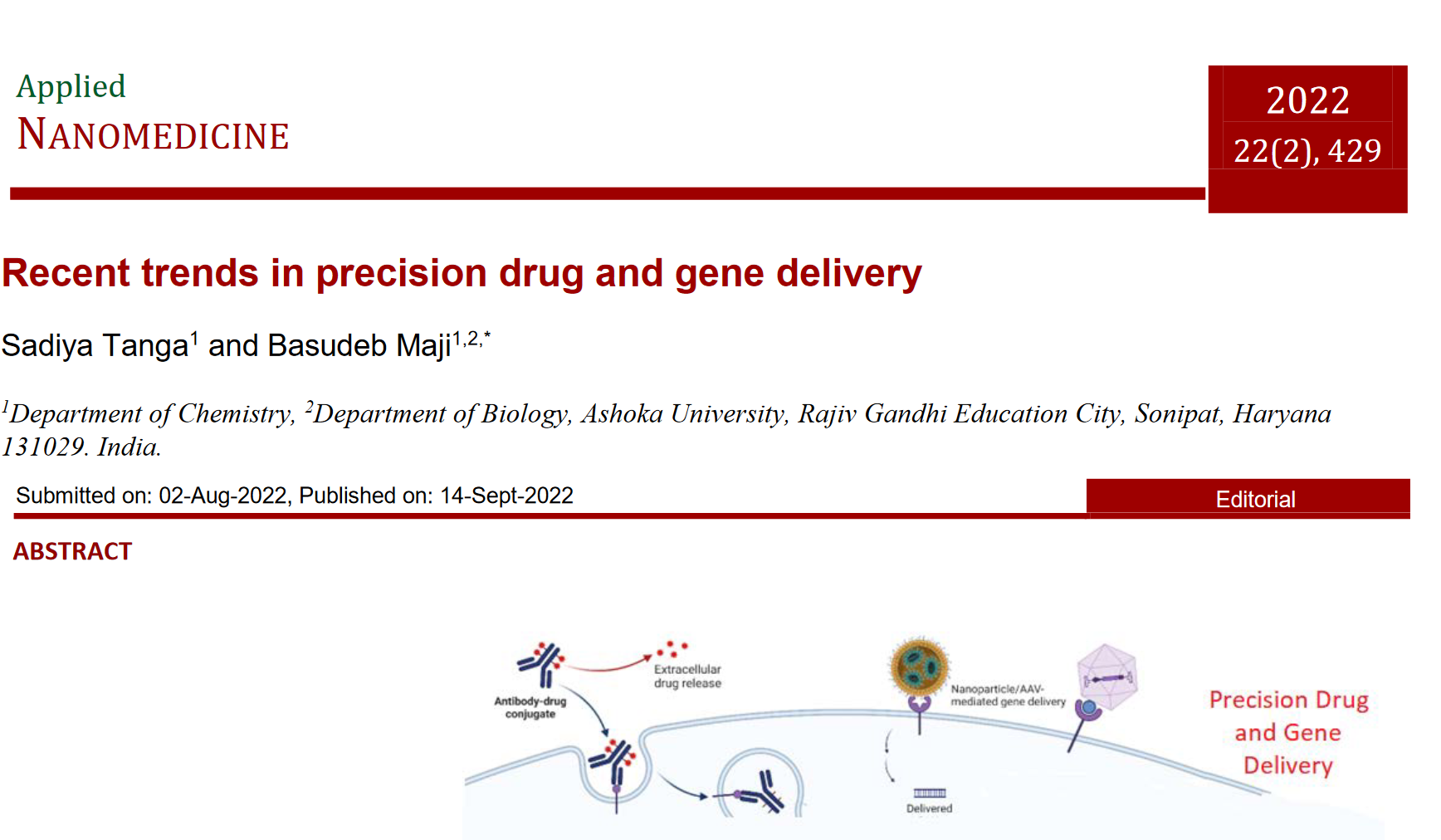
11. Emerging trends in precision drug and gene delivery. Sadiya Tanga and Basudeb Maji. Applied NANOMEDICINE, 2022, 22, 429 (PDF).
12. Sevim Kahraman, Debasish Manna#, Ercument Dirice#, Basudeb Maji, Jonnell Small, Bridget Wagner, Amit Choudhary, and Rohit Kulkarni. Harnessing Reaction-Based Probes to Preferentially Target Pancreatic b-Cells and b-Like Cells. Life Science Alliance. 2021, https://www.life-science-alliance.org/content/4/4/e202000840.
13. Lee#, B Maji#, D Manna#, J Small, B Wagner, A Choudhary. Native Zinc Catalyzes Selective and Traceless Release of Small Molecules in β-Cells. Journal of the American Chemical Society, 2020,142, 6477-6482. # Equal contribution. (IF 14.6)
14. B Maji, SA Gangopadhyay, M Lee, M Shi, P Wu, R Heler, B Mok, D Lee, B Paul, V Dančík, MF Mesleh, A Vetere, LA Marraffini, DR Liu, PA Clemons, BK Wagner and A Choudhary. A high- throughput platform to identify small-molecule inhibitors of CRISPR-Cas9. Cell, 2019, 167, 1067- 1079. Highlighted in more than 12 science media reports. (IF 66.68)
15. D Manna, B Maji, S. Gangopadhyay, and A Choudhary. A singular system with precise dosing and spatiotemporal control of CRISPR-Cas9. Angew. Chem. Int., 2019, 58, 6285-6289. (IF 15.3)
16. SA Gangopadhyay, K Cox, D Manna, D Lim, B Maji, Q Zhou and A Choudhary. Precision control of CRISPR-Cas9 using small molecules and light. Biochemistry, 2019, 58, 234–244. (IF 3.0)
17. MH Kaulage,a B Maji,a S Pasadi, A Ali, S Bhattacharya and K Muniyappa. Targeting G-quadruplex DNA structures in the telomere and oncogene promoter regions by benzimidazole‒carbazole ligands. European Journal of Medicinal Chemistry, 2018, 148, 178-194. aEqual contribution. (IF 5.6)
18. N Dey, B Maji and S Bhattacharya. Motion Induced Change in Emission as an Effective Strategy for Ratiometric Probing of Human Serum Albumin and Trypsin in a Wide Range of Biological Fluids. Chemistry – An Asian Journal, 2018, 13, 664-671. (IF 3.8)
- N Dey, B Maji and S Bhattacharya. A Unique Example of Excitation Triggered Alteration in Sensing Behavior of Fluorescent Organic Nanoaggregates: A Multifaceted Detection Probe for Caffeine in Real- Life Samples. Analytical Chemistry, 2018, 90, 821–829. (IF 6.8)
- B Maji,a CL Moore,a B Zetsche, SE
Volz, F Zhang, MD Shoulders and A Choudhary. Multi-Dimensional Chemogenic Control of CRISPR-Cas9.
Nature Chemical Biology, 2017, 13, 9-11. aEqual contribution. (Highlighted
by Nat. Chem. Biol. News & Views. doi: 10.1038/nchembio.2243.) (IF
12.6)

- MH Kaulage, B Maji, S Pasadi, S Bhattacharya and K Muniyappa. Novel ruthenium azo-quinoline complexes with enhanced photonuclease activity in human cancer cells. European Journal of Medicinal Chemistry, 2017, 139, 1016-1029. (IF 5.6)
- T Hussain, D Saha, G Purohit, A Kar, A Mukherjee, S Sharma, S Sengupta, P Dhapola, B Maji et al. Transcriptional control of CDKN1A (p21/CIP1/WAF1) by TRF2 through the REST repressor complex. Scientific Reports, 2017, 7, 11541. (IF 4.0)
- M Kaulage, B Maji, J Bhat, Y Iwasaki, S Chatterjee, S Bhattacharya, K Muniyappa. Discovery and Structural Characterization of G-quadruplex DNA in Human Acetyl-CoA Carboxylase Gene Promoters: Its Role in Transcriptional Regulation and as a Therapeutic Target for Human Disease. Journal of Medicinal Chemistry, 2016, 59, 5035-5050. (IF 6.2)
- B Maji, K Kumar, K Muniyappa, and S. Bhattacharya, New dimeric carbazole–benzimidazole mixed ligands for the stabilization of human telomeric G-quadruplex DNA and as telomerase inhibitors. A remarkable influence of the spacer. Organic & Biomolecular Chemistry, 2015,13, 8335-8348. (IF 3.6)
- B Maji, K Kumar, M Kaulage, K Muniyappa and S Bhattacharya, Design and Synthesis of New Benzimidazole–Carbazole Conjugates for the Stabilization of Human Telomeric DNA, Telomerase Inhibition, and Their Selective Action on Cancer Cells. Journal of Medicinal Chemistry, 2014, 57, 6973-6988. (IF 6.2)
- B Maji and S Bhattacharya, Advances in the molecular design of potential anticancer agents via targeting of human telomeric DNA. Chemical Communications. 2014, 50, 6422-6438. (IF 6.0)
- B Maji, SK Samanta and S Bhattacharya, Role of DNA Secondary Structures in the Reversible Dispersion/Precipitation and Separation of Metallic and Semiconducting Single-walled Carbon Nanotubes. Nanoscale, 2014, 6, 3721-3730. (IF 6.9)
- B Maji and S Bhattacharya, Molecular design of synthetic benzimidazoles for the switchover of the duplex to G-quadruplex DNA recognition. Chimia 2013, 67, 39-43. (IF 1.2)
- A Paul, B Maji, SK Misra, AK Jain, K Muniyappa and S Bhattacharya, Stabilization and structural alteration of the G-quadruplex DNA made from the human telomeric repeat mediated by Tröger's base based novel benzimidazole derivatives. Journal of Medicinal Chemistry, 2012, 55, 7460-7471. (IF 6.2)
- A Paul, AK Jain, SK Misra, B Maji, K Muniyappa and S Bhattacharya, Binding of gemini bisbenzimidazole drugs with human telomeric G-quadruplex dimers: Effect of the spacer in the design of potent telomerase inhibitors. PLoS ONE, 2012, 7, e39467. (IF 2.7)
- AK Jain, A Paul, B Maji, SK Misra, K Muniyappa and S Bhattacharya, Dimeric 1,3-Phenylene- bis(piperazinyl benzimidazole)s: Synthesis and structure-activity investigations on their binding with human telomeric G-quadruplex DNA and telomerase inhibition properties. Journal of Medicinal Chemistry, 2012, 55, 2981-2993. (IF 6.2)
- AD Tiwari, AK Mishra, SB Mishra, BB Mamba, B Maji and S Bhattacharya, Synthesis and DNA binding studies of Ni(II), Co(II), Cu(II) and Zn(II) metal complexes of N 1,N 5-bis[pyridine-2-methylene]- thiocarbohydrazone Schiff-base ligand. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy, 2011, 79, 1050-1056. (IF 2.9)
Patents
- CRISPR-CAS systems having destabilization domains, US Patent WO/2018/005,873, 2018.
- Inhibitors of RNA guided nucleases and uses thereof. Pub. No.: WO/2018/085288. Publication Date:11.05.2018.
- Inhibitors of RNA-guided nuclease target binding and uses thereof. WO/2020/068304.
- Targeted delivery to beta cells. US patent WO2018195486A1 WIPO (PCT), 2018.
- Methods and compositions for optochemical control of crispr-cas9. WO / 2020/041380, 2018.
- Crispr protein inhibitors, U.S. Provisional Patent Application No. 62/579,727.
Recognition:
- Ramlingaswami Re-entry Fellowship, DBT, 2021
- Invited Speaker at Nature Chemical Biology conference New York University, NY, USA, 2018
- Top 10 Retreat poster of the year 2017 Broad Institute, Cambridge, MA, USA, 2017
- Partners Innovation Discovery Award Brigham and Women's Hospital, MA, USA, 2016
- Postdoctoral Fellow Department of Chemistry, MIT, MA, USA., 2016
- Broad Next10 Research grant Broad Institute of MIT and Harvard, MA, USA., 2016
- Postdoctoral Research Fellow Harvard Medical School, MA, USA., 2015
- Junior and Senior Research Associate Fellowship Indian Institute of Science, Bangalore, India., 2013
- Junior and Senior Research Fellowship, Council of Scientific and Industrial Research (CSIR) and Lectureship Fellowship Ministry of Human Resource Development, Govt. of India., 2008-13
- Postgraduate Academic Excellence Scholarship Foundation for Academic Excellence and Access (FAEA), New Delhi, India., 2006-08
Students:
| Image | Name | Designation | Department | Campus | Contact number | |
|---|---|---|---|---|---|---|
 |
Arkadeep Karmakar | Junior Research Fellow | Division of Molecular Medicine | Unified | arka717@gmail.com | |
 |
Arpita Hota | Junior Research Fellow | Chemical Sciences | Unified | arpitazoology95@gmail.com | |
 |
Mala Thapa | Research Associate | Biological Sciences | Unified | imala.thapa@gmail.com | |
 |
Pallabi Das | Junior Research Fellow | Biological Sciences | Unified | pallabidas925@gmail.com |
Former:
Current Members
1. Sadiya Tanga, Senior Research Fellow, Department of Biological Sciences (Jointly with Ashoka University)

2. Arkadeep Karmakar, Junior Research Fellow, Department of Biological Sciences

3. Arpita Hota, Junior Research Fellow, Department of Biological Sciences

4. Pallabi Das, Junior Research Fellow, Department of Biological Sciences
5. Mala Thapa, DBT RA, Department of Biological Sciences

6. Amaresh Jana, Intern, MSc: Vidyasagar University
7. Koushik Das, Intern, MSc: Vidyasagar University

Alumni
1. Sreehari Dinesh, Project Assistant, Chemistry (2022-23)
BS-MS: IISER Berhampur
Currently: Grad student at Indiana University, USA
2. Dr. Vivek Kumar, Postdoc, Biology (2022)
Ph.D.: Kusuma School of Biological Sciences, IIT Delhi
Currently: Postdoc at Carnegie Mellon University, Pittsburgh, USA
3. Akshara Kulkarni, Undergrad, Biology, Ashoka University (2021-2023)
Currently: MS at University of Oxford , UK
4. Sona J, ASP, Biology, Ashoka University, (2022)
5. Biswadeep Ghosh Roy, Currently: Project Assistant, IISc (2021)
6. Dr. Vipin Rangari,Currently: Postdoc at Washington University (2021)
7. Vaidehi Sharma, B.Sc in Sri Venkateswara College (2021)
8. Dr. Amarnath Pal, Research And Development Specialist, INDO-MIM PRIVATE LIMITED(2022)
9. Ayantika Gosh, Project Assistant (2021-22)
Currently: Grad student at Indiana University, USA
Group News:
Congratulation Sadiya for receiving the DST Travel Grant 2024 to attend the conference in Boston, USA
Congratulation Sadiya for getting selected to participate in the 7th Annual TPD and Induced Proximity Summit 2024 in Boston, USA
Congratulation Pallabi for qualifying CSIR JRF 2024
Welcome to Pallabi, our new member to the group
Congratulation Sadiya for her second research article, this time in Nanoscale.
Dr. Mala Thapa got selected for the prestigious DBT RA Fellowship! Congratulations!